We are investigating cases involving children and infants poisoned by toxic baby foods. Baby food brands Gerber, Beech-Nut, Happy Baby, Sprout Organics, Happy Family Organics, Earth’s Best Organics (Hain Celestial Group), Plum Organics (Campbell’s), Parent’s Choice (Wal-Mart), and Sprout Organic Foods have been found to contain shocking levels of toxic heavy metals such as mercury, lead, arsenic and cadmium. These toxins have been scientifically associated with the development of autism/autism spectrum disorder (ASD), attention deficit disorder (ADD), and attention-deficit/hyperactivity disorder (ADHD) in young children.

A February 2021 report by the U.S. House Oversight and Reform Subcommittee on Economic and Consumer Policy found shocking levels of heavy metals in those major baby food brands listed above. The reports findings were the result of a long-term investigation into the safety of certain brands of baby food sold in the U.S. According to the Report, daily use of these baby foods creates “subclinical decrements in brain function” that “diminish quality of life, reduce academic achievement, and disturb behavior, with profound consequences for the welfare and productivity of entire societies.” Disturbingly, the Report also determined the various manufacturers were aware that their products contained these high levels of known toxins. Both the U.S. Food and Drug Administration (FDA) and the World Health Organization (WHO) agree that heavy metals pose serious dangers to human health, particularly babies and young children.
UPDATE [Oct. 2021] – A new report from the U.S. House Oversight and Reform Subcommittee on Economic and Consumer Policy has found further evidence that baby food products sold in recent years contained significant, dangerous levels of toxic heavy metals.
If your child has been diagnosed with autism, ADHD or ADD after regularly consuming one of the above-named baby food brands as an infant, contact us online or call us at 1-866-252-3535 for a free consultation.


 Paraquat is a highly toxic herbicide used primarily in “no-till” farming operations to control unwanted grass and weed growth. It is also often used along roadways for the same reasons. Most commonly sold under the brand-name Gramoxone by the global agricultural company Syngenta, a subsidiary of the Chinese company ChemChina.
Paraquat is a highly toxic herbicide used primarily in “no-till” farming operations to control unwanted grass and weed growth. It is also often used along roadways for the same reasons. Most commonly sold under the brand-name Gramoxone by the global agricultural company Syngenta, a subsidiary of the Chinese company ChemChina.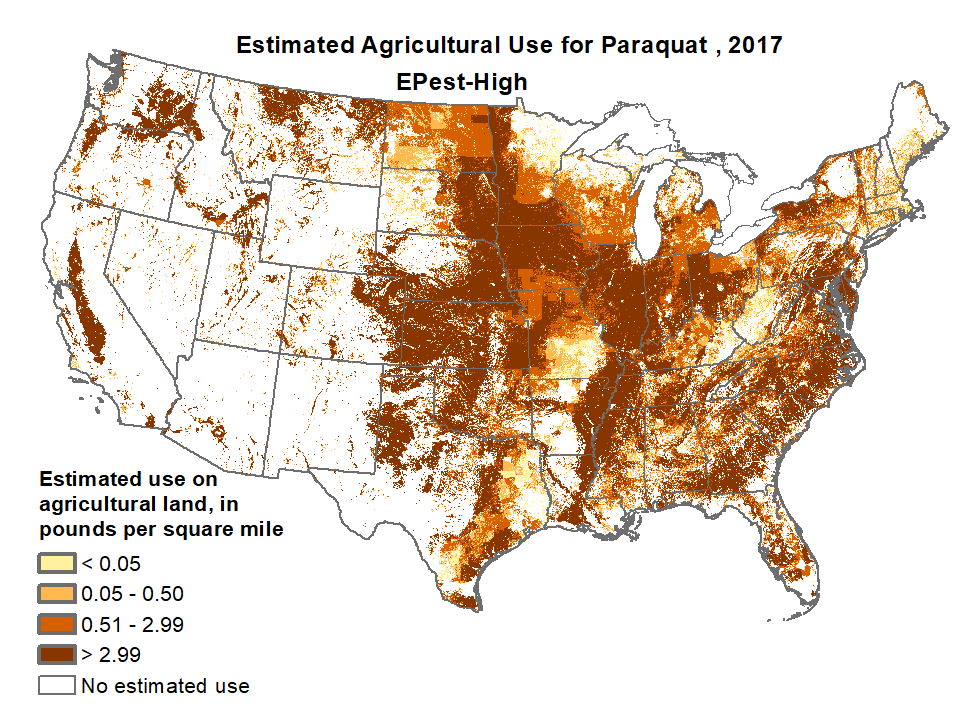
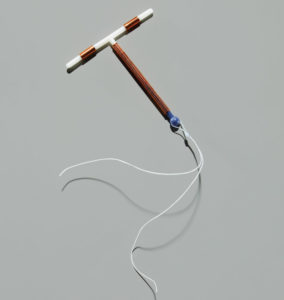 The ParaGard T-380A IUD Birth Control Device has been quite popular. Manufactured by Teva Pharmaceuticals until the fall of 2017, and by CooperSurgical since that time, it is a small, T-shaped device that uses copper to prevent pregnancy as opposed to hormones; the only hormone-free IUD currently on the market. However, “arm-breakage” issues, particularly during removal of the device, have caused thousands of women to undergo surgery to remove the broken arm(s) and repair any associated damage caused by them.
The ParaGard T-380A IUD Birth Control Device has been quite popular. Manufactured by Teva Pharmaceuticals until the fall of 2017, and by CooperSurgical since that time, it is a small, T-shaped device that uses copper to prevent pregnancy as opposed to hormones; the only hormone-free IUD currently on the market. However, “arm-breakage” issues, particularly during removal of the device, have caused thousands of women to undergo surgery to remove the broken arm(s) and repair any associated damage caused by them.
 Scientific studies have established that long-term Elmiron use can cause Maculopathy – a major cause of blindness. A 2018 article published by the American Academy of Ophthalmology addressed a study of six patients who took Elmiron for IC/PBS for at least 15 years. The researchers determined that all six chronic Elmiron users had Maculopathy. A 2019 follow-up by one of the researchers revealed an additional 32 out of a total of 38 patients probably had Maculopathy from chronic Elmiron use, warning of a “potential severe toxicity”. Other studies have corroborated these findings.
Scientific studies have established that long-term Elmiron use can cause Maculopathy – a major cause of blindness. A 2018 article published by the American Academy of Ophthalmology addressed a study of six patients who took Elmiron for IC/PBS for at least 15 years. The researchers determined that all six chronic Elmiron users had Maculopathy. A 2019 follow-up by one of the researchers revealed an additional 32 out of a total of 38 patients probably had Maculopathy from chronic Elmiron use, warning of a “potential severe toxicity”. Other studies have corroborated these findings.