
Zantac (Ranitidine) belongs to a class of medications called H2-blockers. These popular medications are used for treating gastrointestinal conditions such as acid indigestion (‘heartburn’ and ‘sour stomach’) and gastroesophageal reflux disease (GERD) by decreasing the amount of acid produced by the stomach. Zantac was first approved for prescription use by the U.S. Food & Drug Administration in 1983, and in 1986 became the first drug to reach $1 billion in total sales. Zantac became available over-the-counter (“OTC”) (without a prescription) in 1996, and generic versions of Zantac began selling the following year. Despite prolific sales of generic Ranitidine, Zantac sales remain strong: in 2018, it was one of the top 10 antacid tablet brands in the U.S. with sales of almost $130 million.
In September of 2020, it was discovered that when Zantac and other generic Ranitidine products metabolize in the body they produce dangerously high quantities NDMA (N-nitrosodimethylamine), a potent carcinogen. This came to light when Valisure, an online pharmacy, advised the FDA, filing a Citizen’s Petition, that its scientific testing of Zantac and generic Ranitidine revealed that each tablet contained NDMA at levels thousands of times higher than the FDA’s “permissible daily intake limit” (read more below). Notably, Valisure is not just a pharmacy, it operates an ISO (International Standards Organization) accredited laboratory. The FDA considers this accreditation “one of the most important standards for calibration and testing laboratories” and it gives strong credibility to Valisure’s test results.
“There’s no acceptable cancer risk for a drug like this.”
– David Light (Valisure CEO)
Valisure further wrote, “Since 2002, the formation of NDMA by the reaction of DMA and a nitroso source (such as nitrite) has been well characterized in the scientific literature.” In other words, peer-reviewed scientific studies of Ranitidine and NDMA suggest that drugmakers knew — or at a bare minimum should have known — that Zantac (Ranitidine) use exposes users to dangerously high amounts of NDMA.
The dangers NDMA pose to human health are long-standing and well known. It was once used to make rocket fuel and other petroleum-based industrial products. The FDA has established a “permissible daily intake limit for … NDMA of 96 [nanograms].” But according to Valisure, Zantac (Ranitidine) has an NDMA content of between 2.5-2.8 million nanograms per tablet.
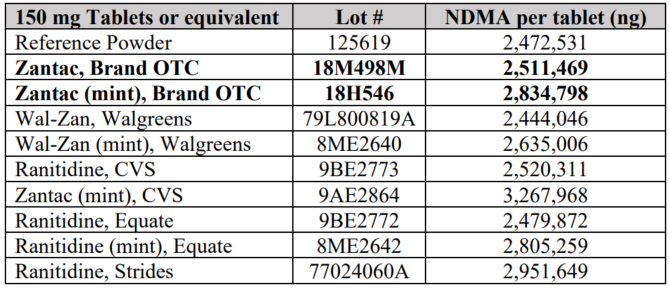
Since the release of Valisure’s report virtually all generic and brand name versions of Zantac have been withdrawn from the consumer market, with most manufacturers voluntarily recalling their products.
Our firm is currently investigating cases where individuals took Prescription and Brand Name Zantac – we are not accepting cases involving generic versions of Ranitidine – before being diagnosed with one of the following types of cancer:
- Bladder Cancer
- Stomach Cancer (including gastric cancer)
- Colorectal Cancer (including colon and rectal cancer)
- Intestinal Cancer
- Esophageal Cancer
- Kidney Cancer
- Liver Cancer
- Pancreatic Cancer
- Uterine Cancer
If you or someone you know has been diagnosed with one of these forms of cancer after taking Prescription or Brand Name Zantac, contact us online or call us at 1-866-252-3535.
Do not stop taking any medication without consulting your healthcare provider.
