OUR FIRM IS NO LONGER ACCEPTING ESSURE CASES
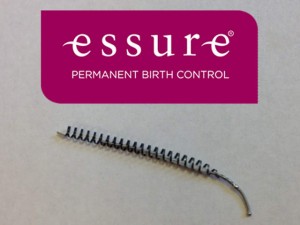
Approved by the FDA in 2002 via the FDA’s “fast track” system of review, Essure is a non surgical permanent form of birth control consisting of a pair of small nickel-titanium coils wrapped in polyethylene terephthalate (PET) fibers. The coils are placed in the fallopian tubes, prompting tissue growth into and around the coils, which blocks sperm from fertilizing a woman’s eggs.
One problem is the nickel-titanium alloy which many, many women are allergic to and causes a plethora of health issues. Worse, reports indicate the Essure device has a tendency to migrate elsewhere in the body after implantation which may result in multiple surgeries to remove the device and/or address any internal damage caused by the device (i.e., perforated or damaged organs such as the uterus and the fallopian tubes).
In June of 2015, the FDA added risk information to it’s website related to Essure. However, the bottom line is that the Essure devices were not properly tested for dangerous side effects before they were ever put on the market in 2002. The FDA has received at least 5,093 adverse event reports since Essure Birth Control was approved in 2002; there were 152 complaints filed in the FDA’s Manufacturer & User Facility Device Experience database in 2012; in 2014 there were 2,259 complaints filed in the MAUDE database, a 1,386% increase. In 2015 there were at least 1,363 MAUDE complaints filed with the FDA.
OUR FIRM IS NO LONGER ACCEPTING ESSURE CASES
